Peptides – chains of amino acids linked by amide bonds – play an indispensable role in the human body. They make up proteins, including enzymes, hormones, and antibodies. One of the limitations of linear peptides is their instability in the physiological environment, where they can be enzymatically cleaved. Scientists are therefore looking for novel ways of improving peptide properties to make them last longer in the body. One such method is “stapling”, which allows the peptide structure to be “locked” in a more stable spatial arrangement using the peptide’s side chains. The staples enhance the peptide’s resistance to enzymatic cleavage and can also be used to introduce other compounds, thus expanding their potential as biologically useful substances. As a new Experientia Foundation stipend award holder, Soňa Krajčovičová is planning to take the development of the peptide-stapling method further during her one-year research stay at the University of Cambridge as a member of Professor David R. Spring’s research group. “To date, peptide stapling has only been used to incorporate a single substance into a peptide. We want to develop a methodology for so-called multifunctional staples, which will enable us to prepare various biologically interesting stapled peptides. With peptide compounds formed in this way, we could for example deliver a drug to a specific target in the body and release it there,” Soňa explains.
Thanks to your stipend from the Experientia Foundation, from July you will be a researcher at the renowned University of Cambridge. How does it feel?
I’m extremely thrilled! The University of Cambridge is a dream come true that would most likely have remained just a fantasy without the fellowship. The Dvořáks, the founders of the Experientia Foundation, are a unique example of good will and modesty, and it means a lot to me that they have decided to support my project. What they have done for Czech science by supporting young scientists in their dreams of an internship abroad and also of running their own research groups is just extraordinary. It will be a huge boost for my career and a wonderful opportunity to move forward, work on my own research ideas, try another area of synthetic organic chemistry, and expand my knowledge of chemical biology. In addition, I’m also really looking forward to being a member of the new group, to new projects and topics, to creative challenges…
If you look at your career path up to this point, what do you think was the most important thing that has led to this moment? What were the most formative experiences?
Actually, I’m not from a family of chemists. When I was at grammar school, I enjoyed all kinds of subjects – political science, psychology, medicine; I was even thinking about studying journalism. The decisive factor for me was having a great chemistry teacher, Mrs Smreková. Thanks to her I found chemistry very interesting and decided to study it at university. During my undergraduate years, I was influenced greatly by another Experientia stipend award holder from 2018, Lukáš Maier, who at that time worked at Masaryk University as a doctoral student in Assoc. Prof. Paruch’s group. He nurtured my enthusiasm for synthetic organic chemistry, as well as for NMR (nuclear magnetic resonance, author’s note), which enriched my knowledge a lot and ended up also guiding my subsequent career path. I was also grateful to him for treating me as an equal partner, even though I was much younger and had little lab experience. He showed me how to do science and how to treat younger students well. Later, as a doctoral student at Palacký University in Olomouc, I learned a lot from my supervisor, Assoc. Prof. Soural, who was constantly giving me very interesting, stimulating and synthetically complex projects, was very approachable, and was a constant source of encouragement to me. The Department of Organic Chemistry in Olomouc was a great environment to work in, and I’m really glad that I was able to complete my PhD there. I don’t think I would be where I am today if I had decided to go to a different institution back then.
You won the stipend with a project in which you will work on peptide stapling. Can you explain what that means in more detail?
Peptides, chains of amino acids linked by amide bonds, are a fascinating class of compounds that play a vital role in the human body and in biological systems in general, because they are the building blocks of proteins, which include the enzymes, hormones and antibodies that are essential for living organisms. However, a major limitation of linear peptides is their instability in the physiological environment, where they can be cleaved by circulating enzymes, making their use as medicines rather limited. For this reason, researchers are looking for ways of improving their pharmacokinetic properties so they could be used in a wider range of applications and at the same time survive longer in the body. One of these methods is what we call stapling, which allows the amino acid side chains to “lock” the structure of the peptide in a more stable spatial arrangement or conformation, making the peptide much more resistant to enzymes (see Figure 1). In addition, staples can be used to insert other substances, such as fluorescent dyes or small biologically active molecules, into peptides, thereby expanding the application and functionality of the peptides in chemical biology.
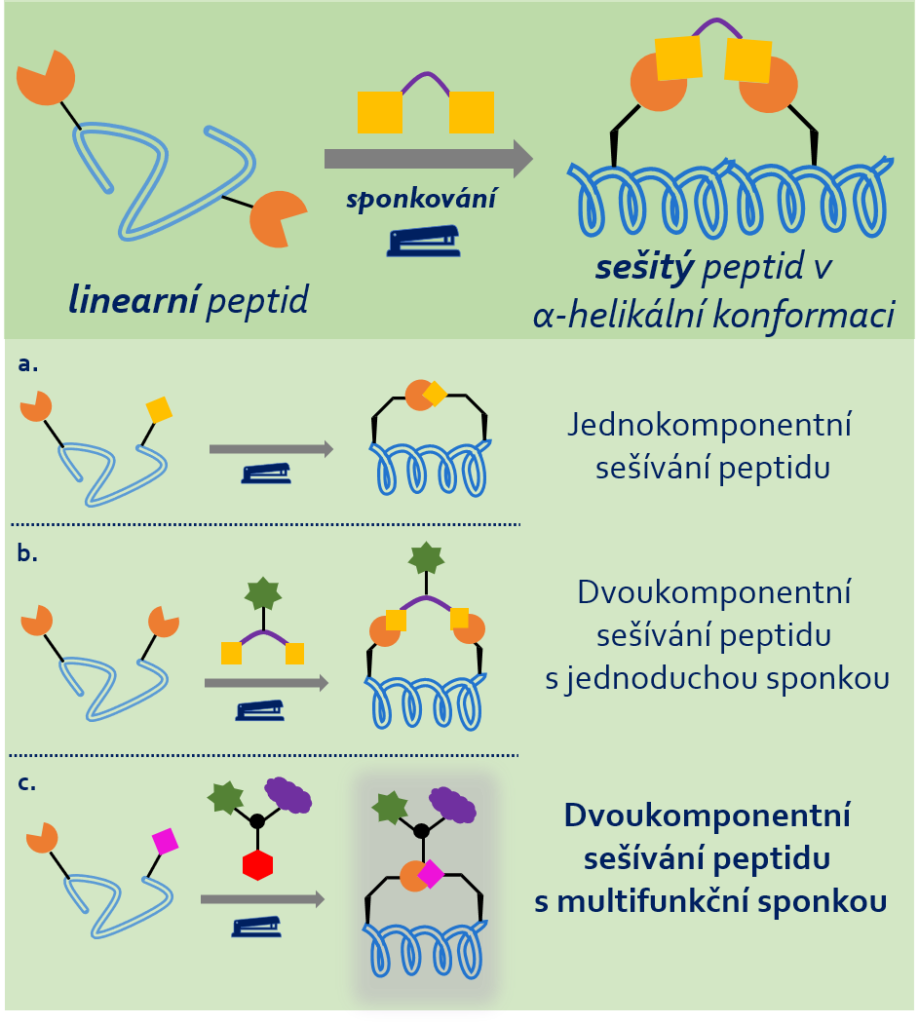
And you are hoping to push the stapling method even further. How exactly?
Yes, I would like to. Up to now, only staples that incorporate just a single useful molecule into peptides have been developed (see Figure 1b), and I would like to develop a synthetic methodology that would allow the preparation of stapled peptide conjugates (complex chemical compounds formed by combining two or more compounds by chemical reactions, author’s note) using a staple that contains multiple substances and is therefore multifunctional. (Figure 1c). The resulting conjugates, in addition to being enzyme-resistant, will be able to selectively suppress certain protein interactions in the human body – those that are mainly associated with cancer – and to deliver and release a drug, e.g. a small organic molecule, to the desired target in the body. This could be a drug that would otherwise cause side effects in the body, because it would also affect the healthy cells (Fig. 1c, purple cloud). In addition, these linked peptides could serve other useful cellular functions, for example by increasing membrane permeability through binding other specific peptides; or they could be labelled with a fluorescent dye and thus be studied by fluorescence microscopy to track their pathway within cells. (Figure 1c, green asterisk). And all this could be achieved by a single stapled peptide conjugate containing a multifunctional staple!
So the key step will be to link the peptide and the multifunctional staple?
Yes, and for the project, I have developed several ideas how to achieve this. Synthesizing organic compounds of such complexity in the lab is not easy, and you have to think carefully about each step of the synthesis. It’s a bit like assembling IKEA furniture without the instructions. You know what you want to build and what it should look like once it’s complete, but you have a million small screws and various other components in the flat pack, and you aren’t sure if they fit exactly where you think they should at first glance. So you keep trying; sometimes it works out the first time round, and sometimes you find out that you put it together the wrong way and you have to go back a few steps to make it fit better into the overall picture. It’s a creative challenge, but I enjoy the process a lot. Later on, I would like to apply my approach to a real system with a proven chemotherapeutic. In general, I like projects and topics with something “meaningful” behind them, i.e. ideally something that might have a possible application, preferably medicinal. Medicinal synthetic organic chemistry and chemical biology are certainly my favourite areas of research, and I see a lot of meaning in them.
Of all the potential topics to investigate in your field, why did you choose peptides in particular?
Peptides, proteins and complex antibody-drug conjugates have fascinated me for years. At about the midpoint of my doctoral studies, there was a period when I was not able to work in the lab for weeks following an injury to my left hand, for which I had to have surgery. It was a difficult period for me because I do love working in the lab, but I used that time effectively – I immersed myself in the literature and began to think about various areas of medicinal chemistry that I might enjoy in the future; I started sorting through my ideas.
So there was a lab accident behind your project? What happened to you?
It happened during my internship in Germany, on a Saturday afternoon, when I was very tired. I wanted to measure an NMR sample, which you put in a thin glass cuvette. The cuvette was cracked at the top, and I didn’t notice. I tried to close it with the plastic cap, but I couldn’t, so I forced it a bit, and the cuvette burst with such force that the shards dug deep into my finger and cut my tendons. I was lucky that the accident happened in a developed country like Germany, where they were able to operate on me quickly. Otherwise, I might not have been able to work with my hands again. So, the idea of working on peptides had been in my mind for a long time. What was needed was the right impulse and finding a research group to give the topic a more realistic shape.
The research group you were looking for turned out to be one headed by Professor David Spring at the University of Cambridge. Why did you choose this group?
Basically, almost from the first year of my doctorate, I knew that after finishing it, I would very much want to go on an internship abroad. I knew I wanted to stay in Europe. I had loved the UK and everything about it (from music and TV series to architecture and football) ever since I went there on a school trip, and I thought it would be great to try and find an internship there, because UK universities and science are considered the best in the world. In addition, my partner relocated from Tokyo to London for his work at the same time, which I also took as a good sign. So I did some really thorough research into all the UK universities with good chemistry departments, including all potential research groups, which took me about 3–4 months. I remember very well the moment I came across Professor Spring’s group. I was taking the train home to Slovakia and was browsing through the website of the Department of Chemistry at the University of Cambridge, where I read that Professor Spring investigates protein-protein interactions and the medicinal chemistry of peptide drug-and-antibody conjugates. My eyes immediately lit up, my heart began to pound, and I knew this was it.
Really?
Yes, and when I went over Professor Spring’s profile in more detail, I immediately felt that we had common scientific interests and that I would really like to go and work with him. So, few days after completing my doctorate, I wrote him an email. He invited me to Cambridge to meet the group and present my research to date. I prepared well for the meeting: I read his publications and studied the group’s website in detail to get as much of the background picture as possible. I had lunch with his group and had the opportunity to attend three group meetings. At the end of the day, I was able to choose the area that interested me most. I chose peptides and biotherapeutics and then went back home to study the literature and design a project. Professor Spring had sort of thrown me the bait and I rose to it and developed the project in such a way that it would fit into his group’s portfolio, but at the same time it was something that no one else had done before. In addition, after seeing the presentation of my results to date, he told me that I would be a “perfect fit” for his group, and that also motivated me a lot to secure the funding and go to Cambridge.
You will be starting your stay in Cambridge as early as July 2021 – what are you looking forward to the most?
Being in a new lab and having my own fume hood! I’m looking forward to working on the project and making my ideas a reality in the lab. New beginnings are difficult everywhere, but I’m sure that I will be able to integrate into the group quickly. At the same time, I feel a tremendous amount of respect for the prestigious university and for this whole opportunity, which few people get, and I’m certainly not taking it for granted. In the UK I will try to spread not only the good name of the Experientia Foundation, but also of the Department of Organic Chemistry in Olomouc, and I will do my best to achieve this.
You were saying that you love working in the lab. Tell me, though, how do you like to take a break from lab work?
I am not able to rest properly, to be honest, but I do enjoy running, and during the lockdown I also used to take long walks, as much as 15km. I would listen to podcasts on my walks, and not just on science; I really like Deník N, Respekt and Vinohradská 12. My guilty pleasure is also the Czech TV news channel CT24, which is on 24/7. And I also like to watch TV series: I have subscriptions to several streaming services, and I can easily go through six series in three weeks if I’m hooked enough. For example, I’m currently bingeing on Downton Abbey, trying to tune my ear to British English, which I will need a lot from now on.

Soňa Krajčovičová
was born in 1991 in Bratislava. In 2010, she started her bachelor’s degree in biochemistry at Masaryk University in Brno, where she discovered a passion for synthetic organic chemistry and decided to continue in it with her master’s degree at the same university. In 2015, she moved to the laboratory of Assoc. Prof. Miroslav Soural at Palacký University in Olomouc for her PhD, where she prepared new biomolecules based on inhibitors of protein kinases and triterpenes as potential antitumor agents. During her studies, she also became an NMR operator at the Department of Organic Chemistry in Olomouc, where she was in charge not only of the smooth operation of the instrument but also of measuring and evaluating more complex spectra for her colleagues. She completed her doctorate in organic chemistry in 2019 and remained a member of the group as a postdoctoral researcher. Thanks to the CZK 945,000 stipend from the Experientia Foundation, in July 2021 Soňa will travel to the University of Cambridge for a one-year research stay in Professor David Spring’s research group.